It's really not worth too much effort to try to address fossil fuel advertising in a three card Monte systems with something called science, but even a fool with no science education whatsoever can understand it takes energy to run a microwave.
The full original paper (as opposed to a journalistic representation subject to misinterpretation) is here: Dongkyu Lee, Jaemin Yoo, Gunsu S. Yun and Hyungyu Jin J. Mater. Chem. A, 2024,12, 33526-33536
Notably, one of the authors, Dr. Gunsu Yun, is from the Division of Advanced Nuclear Engineering, Pohang University of Science and Technology. Our fossil fuel salespeople trying to rebrand fossil fuels at hydrogen here and elsewhere, like most advocates of continued fossil fuel dependence run around expressing their hatred of nuclear energy, since unlike hydrogen, it is primary energy and thus capable of eliminating dependence on fossil fuels.
The paper represents an effort to reduce the required temperatures for the thermochemical production of hydrogen, presumably for captive use, using primary nuclear energy, thus lowering the enthalpy and entropy penalty of making hydrogen from water, although the penalty exists, as it must from the laws of thermodynamics.
The authors explicitly state the magnitude of the energy losses for the production of hydrogen in the process.
Since DU lacks an equation editor, I'll post the relevant text as a graphic object:

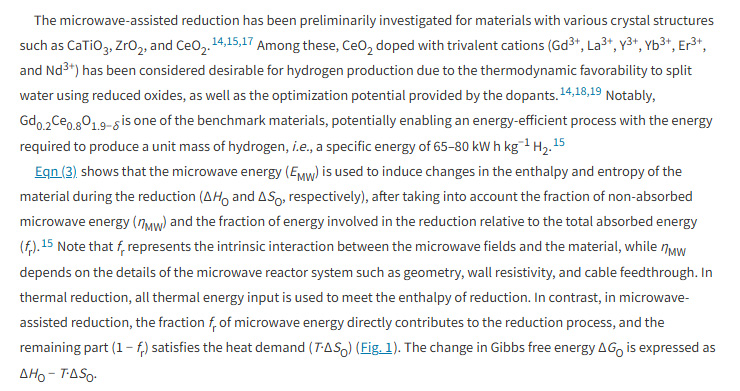
The energy required to produce a kg of H2 is given as between 65 kWh/kg and 80 kWh/kg, which translates into 234 MJ/kg and 284 MJ/kg.
A kg of H2 has a heating value (depending on the thermodynamics of the device in which it is consumed) of between 120-142 MJ/kg.
Thus at the high end, the energy lost in this scheme to waste energy to make hydrogen - the purpose of which is uniformly to greenwash fossil fuels - is 140 MJ/kg and at the low end, 117 MJ/kg.
The laws of thermodynamics are not subject to being overturned by marketing. They are inviolable laws of physics, as well the authors understand. They're scientists, not marketeers trying to greenwash fossil fuels.
What they have not explicitly discussed is that electricity is required to run a microwave, and electricity, by its very nature is a thermodynamically degraded form of energy.
The only value of this process would be a situation where excess electricity was produced in off peak hours as a result of process intensification to recover exergy from nuclear heat.
Otherwise, thermochemical cycles avoiding electricity will always be thermodynamically superior, since it is far more direct, but no matter how hydrogen is made, it destroys exergy, the recoverable energy of a process.
Have a nice evening.
 = new reply since forum marked as read
= new reply since forum marked as read

