Science
Related: About this forumPossible improvements in the electrowinning of the lanthanide elements (aka "rare earths).
Last edited Wed Apr 10, 2024, 08:47 AM - Edit history (1)
I have very little time tonight, and am way, way, way behind on catching up on my reading, but I thought I'd drop a note on processing neodymium, a key metal in electric motors, generators and other devices, which is often described as being a critical material is the so called "green revolution" or "energy transition" that is frequently hyped but are of little consequence.
I'll simply refer to this paper, and excerpt it a little, to show how "green stuff is actually made, coupled with a proposed improvement which may or may not ever be industrialized.
The paper is this:
Sustainable and Energy-Efficient Production of Rare-Earth Metals via Chloride-Based Molten Salt Electrolysis Benjamin Holcombe, Nicholas Sinclair, Ruwani Wasalathanthri, Badri Mainali, Evan Guarr, Alexander A. Baker, Sunday Oluwadamilola Usman, Eunjeong Kim, Shohini Sen-Britain, Hongyue Jin, Scott K. McCall, and Rohan Akolkar, ACS Sustainable Chemistry & Engineering 2024 12 (10), 4186-4193
Presently, REE metal production involves “molten salt electrolysis (MSE)” to convert oxide feedstock into metal. The state-of-the-art method for Nd metal processing uses an oxyfluoride MSE process in which Nd2O3 is dissolved in a molten NdF3–LiF mixture at a temperature exceeding the melting point of Nd (1050–1100 °C). Utilizing a carbon anode, Nd2O3 electrolysis leads to the following overall cell reaction: (4)
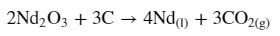
This method has several drawbacks, which prohibit its widespread deployment. (5−8) The process necessitates the oxidative consumption of a carbon anode, producing carbon dioxide (CO2), a greenhouse gas (GHG). Annual global production of about 70,000 tonnes of Nd results in the emission of about 16 thousand tonnes of direct CO2. (9) Although this rate of CO2 emission is small in comparison to other metal production processes (e.g., Al or steel production), advancements in electric motors and the push toward green energy is predicted to increase Nd demand by at least fourfold by 2030 with steady growth thereafter. Another disadvantage of oxyfluoride electrolysis is that at high current densities, the fluoride ions produce perfluorocarbons (PFCs), such as CF4 and C2F6 at the anode, which are GHGs with very large global warming potentials (GWPs). Over time, the “consumable” carbon anode uncontrollably increases the interelectrode gap and thus the cell voltage, making process control and scale-up difficult, as well as increasing energy consumption. Finally, the consumable anode necessitates “batch” operation wherein significant energy is wasted in cell heat-up and cool-down cycles, lowering process energy efficiency. These drawbacks make oxyfluoride MSE unattractive for scale-up from a safety, cost, or sustainability point-of-view in geographical locations with stringent environmental regulations...
The authors propose dissolving the oxide in HCl and electrorefining NdCl3 in an KCl/LiCl eutectic. The chlorine is oxidized on a "dimensionally stable titanium electrode coated with RuO2.
Neodymium is a component of used nuclear fuel and can be recovered from it for use. Many molten salt proposals have been made for the recovery of valuable elements from used nuclear fuel, and this technique might merit in some part of the process, being put to use for recovery of fission product lanthanides, notably lanthanum, cerium, praseodymium and neodymium. Thereupon the recovered elements could be sold to industy.
Have a nice day tomorrow.