Science
Related: About this forumVanadium and Iron Only Bacterial Nitrogenases.
I won't have much time to discuss this paper, but I came across it in the "you learn something every day" category.
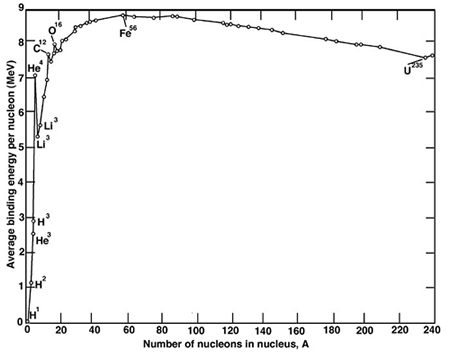
I have always assumed that all enzymes for nitrogen fixation coordinate molybdenum, molybdenum, along with iodine, being the only 5th period elements in the periodic table that are essential for life. This is somewhat surprising, since these elements occur after iron in the periodic table, iron-56 being the most stable transition element in terms of binding energy. Elements beyond the binding energy, although they form by the S-process in stars, tend to be rare relative to the light elements, in particular carbon, hydrogen, oxygen and nitrogen, that dominate living systems, along with other elements like sulfur. The chief transition elements that are important in life all appear close to iron in the periodic table, iron, of course, as well as copper, zinc, & cobalt.
The binding energy curve:
This paper indicates that my assumption about molybdenum being essential to life is wrong:
Caroline S. Harwood Iron-Only and Vanadium Nitrogenases: Fail-Safe Enzymes or Something More? 2020 J Annual Review of Microbiology 247-266 74 1
Until now, I haven't heard much about the biological properties of vanadium.
Some excerpts:
The introduction:
Figure 1:
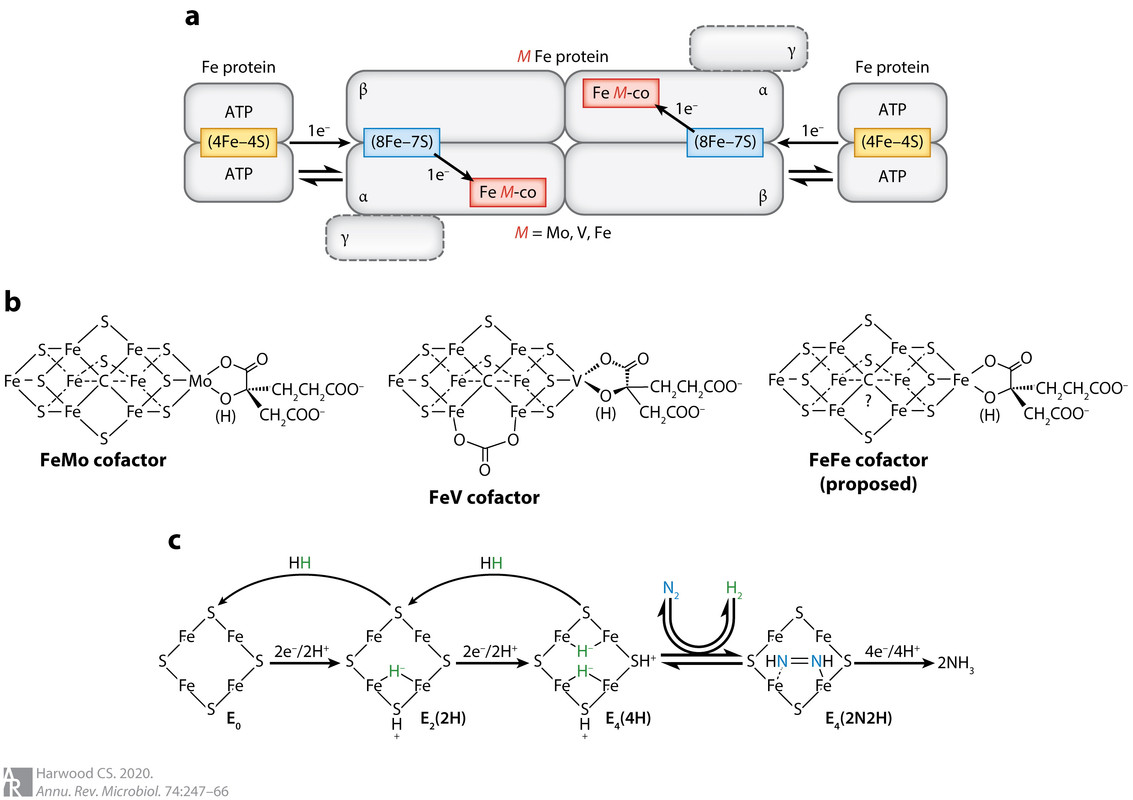
The caption:
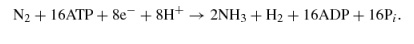
From this formulation one can see that nitrogen fixation is an ATP-intensive reaction, and it also requires large amounts of protons and reductant in the form of energy-rich electrons. N2 reduction to NH3 is difficult to achieve because although it is thermodynamically favorable, there is a large activation energy barrier to catalysis that must be overcome (90) (Figure 2). Because of the complexity of the reaction it catalyzes, nitrogenase has a slow turnover rate (104). For these reasons it needs to be synthesized in large amounts, in some cases as much as 10% of total cellular protein (20), to sustain growth under nitrogen-fixing conditions.
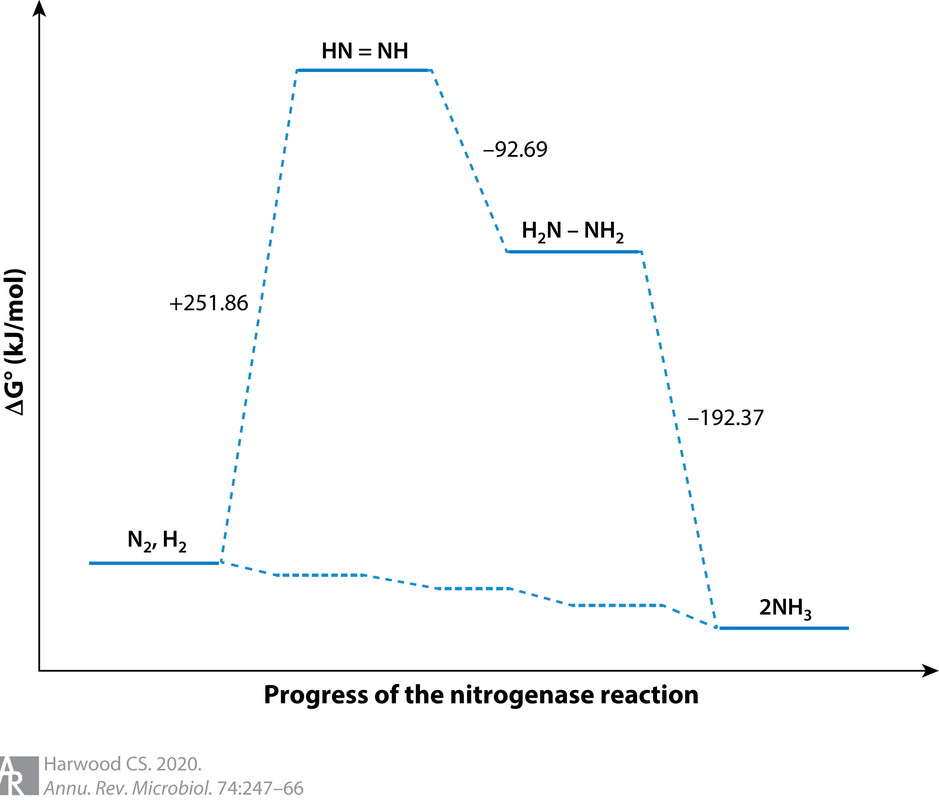
The caption:
Based on recent studies (32, 33), the reactions catalyzed by the alternative nitrogenases are as follows:
Fe-only nitrogenase,
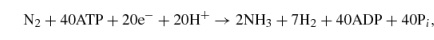
V nitrogenase,
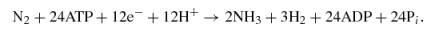
All three nitrogenases produce hydrogen gas (H2) as an obligate aspect of nitrogen fixation. However, the alternative nitrogenases, and in particular Fe-only nitrogenase, produce much more H2 per N2 reduced than does Mo nitrogenase. This has led to the suggestion that nitrogenases might be used to generate H2 as a biofuel, and there have been a large number of studies directed toward this application (30, 59, 62, 78, 93).
Ain't gonna happen, the biofuel, but anyway...
It appears that these nitrogenases are found together, and are regulated according to the availability of molybdenum.
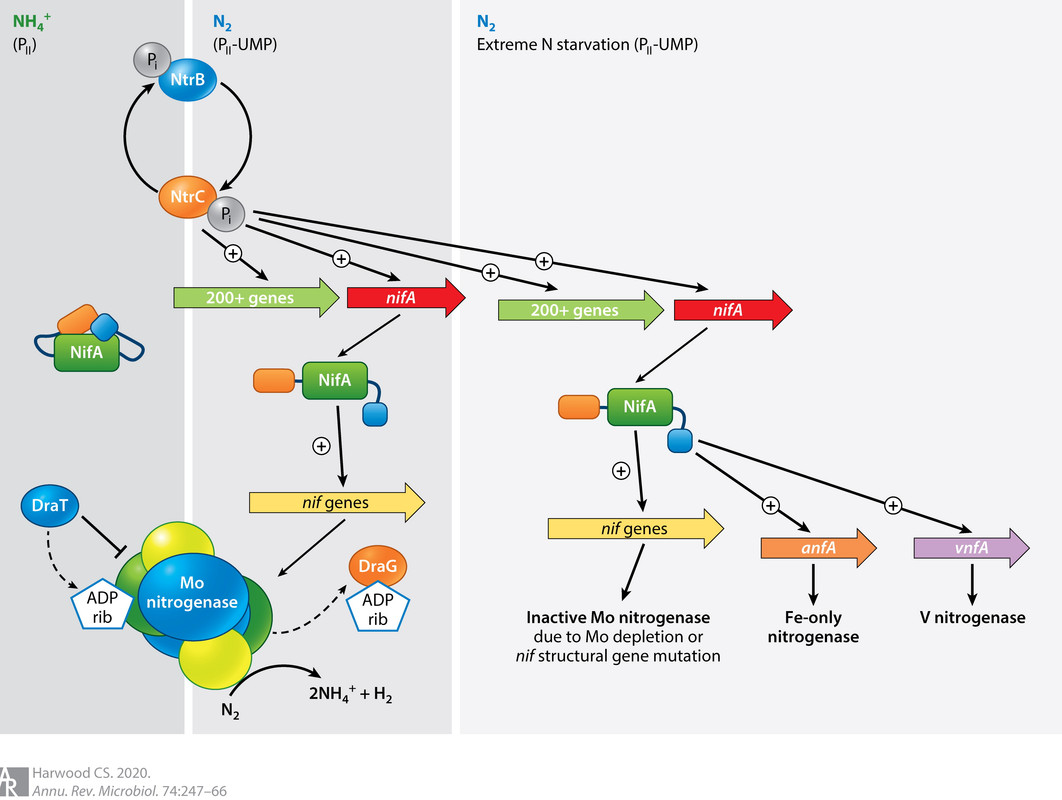
As it happens, right now on this planet, nitrogen fixation is largely an industrial process, the Haber-Bosch process, which represents an environmental challenge owing to the downstream growth of N2O gas, laughing gas, no laughing matter as it is both a greenhouse and ozone depleting gas. The Haber-Bosch process represents the second largest application for industrial hydrogen, after oil refining, despite all the tiresome bullshit one hears all the time about hydrogen cars and other exercises in wishful thinking.
Esoteric but cool.
eppur_se_muova
(37,348 posts)I didn't actually know that H2 cogeneration was always part of the process. Makes me wonder how meaningful that energy diagram is (although it does make the point about the strong N≡N bond) -- what are the intermediates between N2 and N2H2 ?? Nothing about adding protons and electrons to N2 makes it obvious that H2 should always form.