Environment & Energy
Related: About this forumHigh-Temperature Ferrofluids Based on Molten Salts
The paper I'll discuss in this post is this one: High-Temperature Ferrofluids Based on Molten Salts, Phillip W. Halstenberg, Ellie M. Kim, Dianna Nguyen, Dmitry Maltsev, Dustin A. Gilbert, and Sheng Dai Industrial & Engineering Chemistry Research 2025 64 (1), 429-440.
The paper was written by scientists at Oak Ridge National Laboratory and the associated University of Tennessee. Oak Ridge National Laboratory is the place where the first (and moderately famous) experimental thorium molten salt reactor (MSRE) was built and operated. There is some enthusiasm - actually a fair amount - of bring commercial examples of this reactor forward, although there have been huge advances in technology since then.
The MSRE used a eutectic salt called "FLIBE," a eutectic mixture of lithium and beryllium fluorides. This paper refers to a different class of eutectic salt which are chlorides, not fluorides, a topic on which I'll briefly comment below.
From the introduction:
Methods to optimize the specific heat capacity (C[sub[p) of molten salts are currently an area of research focus, including the current work. Addition of nanoparticles to molten salt can form a stable colloid, or nanofluid, and increase the C[sub[p of the fluid. (9-12) The increase is attributed to a new semiordered phase that is more dense than the base fluid and enhances the molten salt’s C[sub[p. (13,14) This new phase is semisolid and forms due to strong interactions between the nanoparticle and solution. (15) While preparing for this work, it became apparent that there are relatively few published works which examined the impact of adding metallic nanoparticles to molten chloride salts and particularly the potential of high-temperature magnetic properties. It has been shown extensively that the addition of magnetic nanoparticles to solutions can dramatically affect a variety of bulk solution properties. (16-26) Thus, the overarching goal of this work was to create and investigate the first molten metal chloride salt based ferrofluid.
Ferrofluids, first produced by NASA in the 1960s, contain magnetic nanoparticles that are suspended in a liquid matrix that causes the entire solution to become responsive to an external magnetic field. (27) Previous works have prepared ferrofluids by suspending nanoparticles, typically 1.0–10.0% weight, in aqueous or organic base fluids and using surfactants to modify the surface of the nanoparticles, preventing agglomeration and stabilizing the suspension. (28,29) The use of aqueous and organic base fluids has limited the operational temperature. Even so, ferrofluids have found many applications, including use as heat transfer fluids related to small electronic devices. (30) Most applications have been microscale, although scaling to industrial applications is possible. (31)
The lack of research effort devoted to high-temperature ferrofluids could be due to the relatively low Curie temperature (T[SUB[C) of most magnetic materials. Upon reaching T[SUB[C, thermal effects dominate over the magnetic coupling, resulting in a loss of long-range magnetic polarization. (32) A challenge with ferrofluids is the smaller particles tend to result in a more stable colloid, but the thermodynamic stability of the magnetic ordering and saturation magnetization of the nanoparticles scales with the particle volume, thus there is an inherent dichotomy between suspension stability and magnetic ordering. (33−35) Previous calculations of ferrofluid stability while exposed to an external magnetic field showed that maximum colloidally stable particle size increases as temperature increases. (36) Building on this result, a high-temperature molten salt ferrofluid should support a stable colloidal suspension of larger nanoparticles with a correspondingly improved thermal stability. In this way, high-temperature ferrofluids remain a possibility.
Altogether, the no-contact control of the thermophysical and magnetic properties in ferrofluids presents a unique opportunity for a new class of magnetic high-temperature HTFs with applications beyond nuclear reactors and concentrated solar power systems. We propose that the candidate ferrofluid must demonstrate three qualities to be practically deployed: (1) chemical stability at operational temperatures, (2) sufficient response to applied magnetic fields, and (3) colloidal stability of the suspension. This work evaluates the first two requirements by experimentally examining two molten metal–chloride salt ferrofluids for potential application as high-temperature HTFs....
All ferromagnetic metals, including the transition metals, iron, cobalt and nickel (as well as certain lanthanide alloys) exhibit a "curie point" at which they lose their magnetism. The point of this paper is to overcome that issue to make molten salts that can be magnetically propelled. The salts discussed here use cobalt, a ferromagnetic element, a metal that is mined by effective slaves, the property of Elon Musk, slave holder, in the "Democratic Republic of Congo" for use in "lithium ion batteries" for electric cars and other applications, such as laptop computers.
People here used to celebrate Elon Musk because of his stupid electric car and his Powerwalls® the latter of which were supposed to make the useless so called "renewable energy" affectation reliable, which of course, it will never be. I have seen people here wax stupid over Powerwalls®, inspiring me to comment on their viability as a solution to Dunkleflaute.
The Number of Tesla Powerwalls Required That Would Address the Current German Dunkleflaute Event.
The whole Musk/electric car/Powerwall® shitshow is transparently a failure. The planet is in flames, physically, morally, and politically.
(Another note on Musk: Discussions of human slavery will probably get you banned at "X," a right wing propaganda promotional site. I wouldn't know; I wasn't involved, even when it was "twitter." If I weren't an atheist, I'd thank God.)
In steel nuclear reactor cores, small amounts of iron are transmuted into cobalt, although in tiny amounts. Cobalt so obtained will always be radioactive, because of the accumulation of 60Fe, a long lived radioisotope that decays to 60Co, in addition to 59Co, the single nonradioactive isotope of the element, which is generated from the short lived radioisotope of iron, 59Fe. t1/2 44.495 days. This short half-life precludes much 60Fe being formed from it, although tiny amounts will be so generated. There is, regrettably, not much cobalt generated. In any case 60Co is a very useful radionuclide with many industrial, scientific, and medical uses. Its half life is reasonably short: t1/2 = 5.271 years. This useful nuclide can be leached from iron containing 60Fe, t1/2 = 1.5 million years. Iron isotopes can be facilely separated using ultracentrifuges and volatile carbonyl complexes.
In any case, to return to the paper, the authors experimented with a variety of molten salt compositions to address the issue of maintaining magnetic stability.
The following graphics show the composition of some of these salts and the effects on cobalt magnetization.
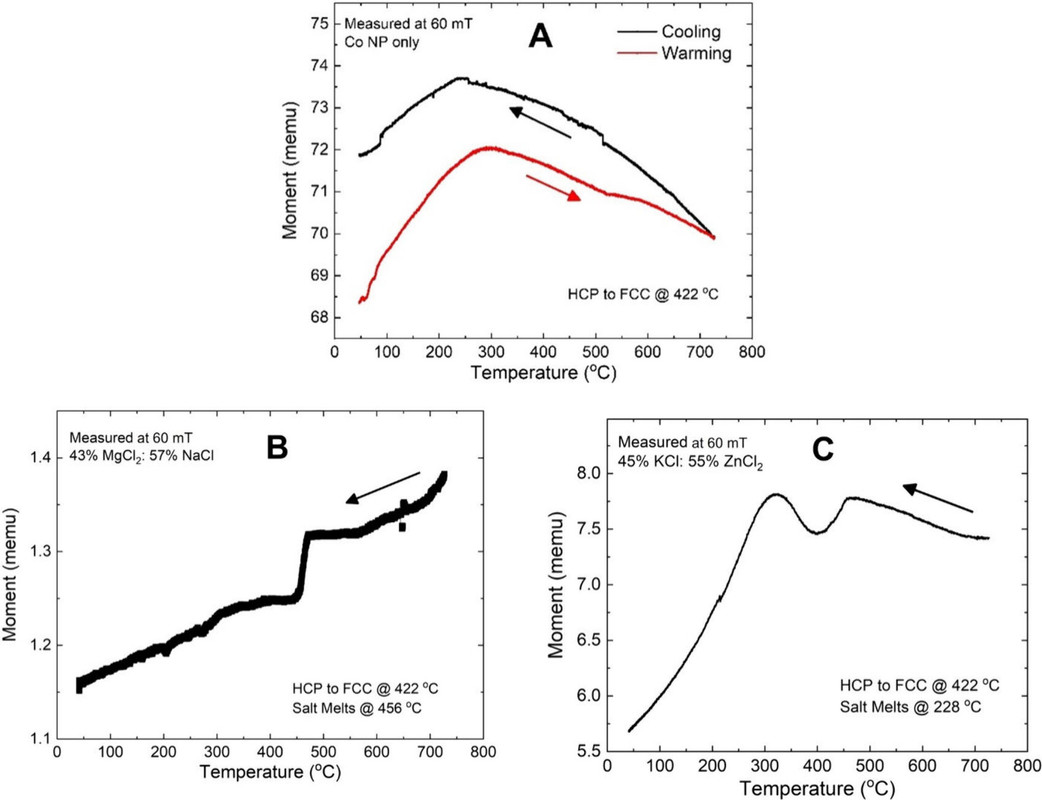
The caption:

The caption:

The caption:
Figure 9. Temperature dependent magnetic force of 25–30 nm cobalt nanoparticles at varying concentrations in a NaCl–MgCl2 eutectic mixture. Data are normalized to the force measured at room temperature (approx. 30 °C).
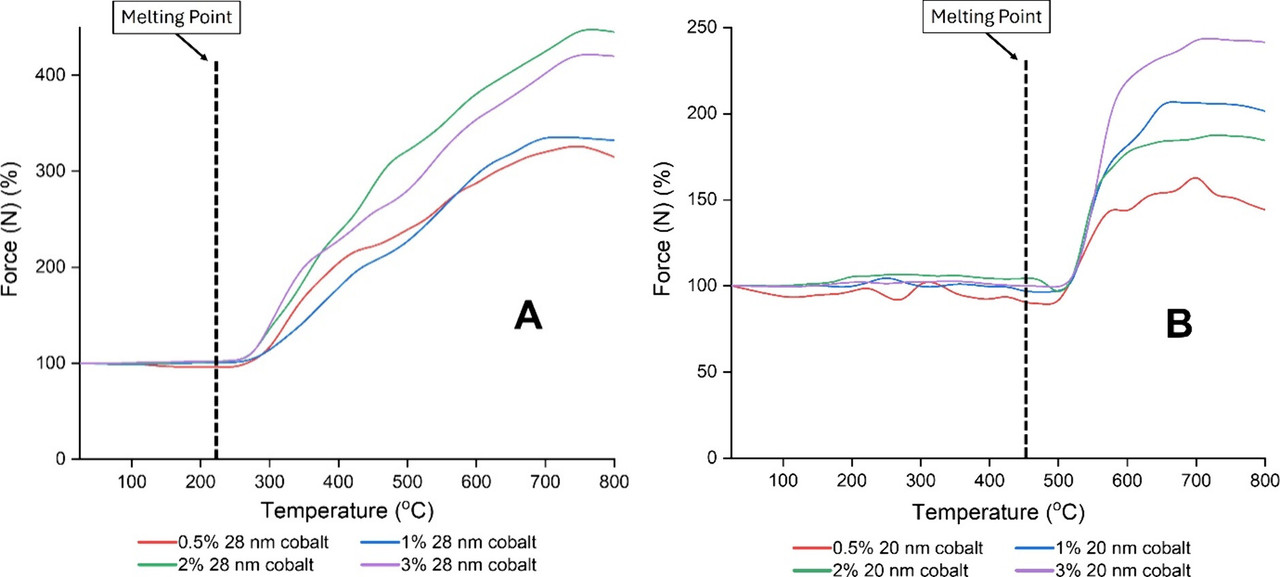
The caption:
A disadvantage of chloride salts in nuclear reactors, is that chlorine has two isotopes separated by 1 amu, 35Cl and 37Cl. The former will capture neutrons, albeit with a small neutron capture cross section in the fast neutron spectrum weak resonances as shown in the following graphic:
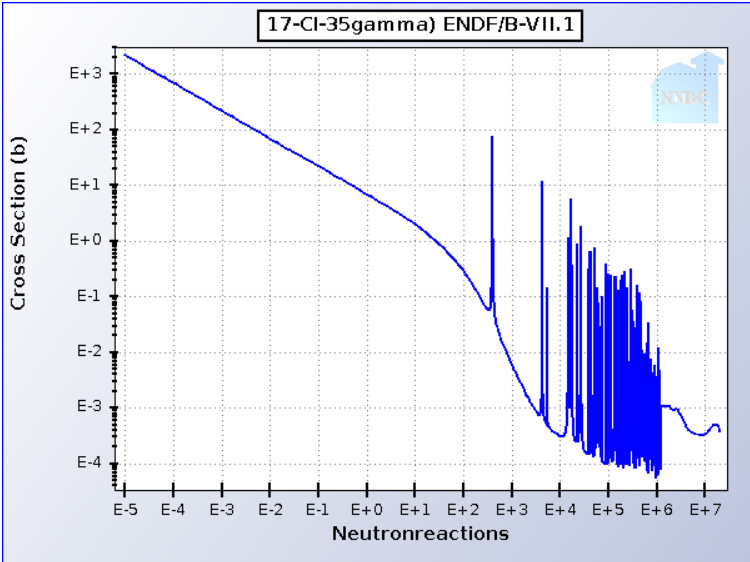
Evaluated Nuclear Data File (ENDF) Retrieval & Plotting
The generated 36Cl has a long half-life (t1/2 = 301,000 years) and a very low neutron capture cross section across the energy spectrum - it's pretty much transparent to neutrons- and thus would need to remain in use for a very long time. It's not an insurmountable problem to my mind, but will generate, to be sure, angst among radiation paranoids whose selective attention has driven extreme global heating.
The advantage of neutron transparent nuclides is neutron efficiency, so access over long time periods to 36Cl is not necessarily a bad thing.
From the paper's conclusion:
It's an interesting paper, published just before the fall of the United States in one of its premier national laboratories. It's all very sad.
Try to have a nice weekend as much as you can as your country disintegrates.
Oneear
(362 posts)If you would want to come Just anytime to start and haul it off
NNadir
(35,057 posts)...of saving the world, something that is increasingly a long shot.
There is a lot of thorium around the world, much of it in mine tailings from the lanthanide mining industry. It should be recovered and used.
StevieM
(10,559 posts)And, specifically, that you were concerned about the toxicity of beryllium. Does this mean that you are warming up to molten salt reactors?
NNadir
(35,057 posts)...a fluoride anion.
This is a different salt than Flibe, one that doesn't contain beryllium.
It does contain chlorine, which is somewhat problematic because it has two stable isotopes with a long lived radioactive isotope between, 36Cl.
In theory, albeit not in practice, there are an infinite number of molten salts, including organic molten salts know as ionic liquids.
I'm actually working to get my son to listen to a mixed liquid metal/molten salt hybrid.
John ONeill
(66 posts)Hi Nnadir,
Do you think this might work for fluorides? Copenhagen Atomics are running a non-fission prototype with 700C fluorides (with lithium and uranium, no beryllium, heavy water moderator presumably replaced by light water for now.) They are starting a one year non-stop trial. The reactor has seven high-temp pumps in it, which they have developed and been testing for some time, but five year planned no-maintenance runs at that temperature sounds tricky.
John O'Neill
NNadir
(35,057 posts)To be a nice guy, I looked at it. The reason for so doing was concern about the accumulation of tritium. (It turns out that tritium will be in short supply if all those fusion toys start running.)
To be perfectly honest, I have lots of plutonium/salt phase diagrams, and I'm sure somewhere in my files, the Flibe/ThF4, UF4 phase diagram, but I don't seem to have a UF4/LiF phase diagram. If they're doing this at 700°C there must be a eutectic since this is lower than the melting point of pure LiF.
From my perspective, fluoride is the preferred halide anion in fission settings because of it is monoisotopic, has a very low neutron capture cross section, and no long lived radioisotopes. Both bromine and iodine have high neutron capture cross sections, and as mentioned above in the OP and posts, chlorine has two isotopes and a long lived radioactive isotope between them.
That said, cesium iodide and rubidium iodide have some remarkable physical properties which suggest to my mind many nuclear applications, bromides not so much. Both CsI and RbI are fission products, which makes them fun; the former being more fun because it will always be highly radioactive in the form fission product, which to my mind suggests a tremendous value.
Can the Copenhagen thing work? Sure, I'd guess, I don't see a reason it wouldn't. But a five year test is a long time, given the scale and magnitude of the crisis of extreme global heating. (This is triply true for the magical success of fusion reactors.) I think better options are currently available for immediate or near term build.
I hope this helps.
John ONeill
(66 posts)To clarify, the 5 years is intervals between swapping out the core, once commercial. Fission testing at 1 MW thermal is due this year, at the Paul Scherer Institute in Switzerland; the one year test will be on one of their other test rigs in Denmark.
Thanks for the response, interesting as always.
NNadir
(35,057 posts)...age of nuclear creativity, with lots of start ups generating a plethora of ideas. These will not pan out.
By way of history, there once was a plot to assassinate Werner Heisenberg at the Paul Scherrer Institute during World War II, to be carried out by a former major league catcher, Moe Berg. The idea was to disrupt a putative German nuclear weapons program that the West assumed was underway; actually one wasn't underway, although some primitive reactor technology was being explored. The assassination didn't come off, obviously. Denmark was peripherally involved, since it was the home of Niels Bohr, who had discussed nuclear issues with Heisenberg in occupied Denmark before escaping (he was half Jewish) to the West.
If nothing else, running the described reactor at 1 MW should generate at least some tritium for the fusion toys. It's a good thing to try out I think. I wouldn't personally invest in it, but I wish them every success.
My mistake, they're using FLiNaK.
NNadir
(35,057 posts)I'm not really a molten salt kind of guy anymore: I'm a liquid metals kind of guy, I guess, but FLINAK is, in my view, with some limitations, a wonderful molten salt inasmuch as it supports the fast neutron spectrum, critical to extension of uranium resources essentially to infinity.
John ONeill
(66 posts)From Wikipedia article -
'The company describes its reactor core as shaped like an onion {2.5 metre diameter}, with the outermost layer being a breeding blanket of 2,000 liters of LiF and ThF4 salts at 600°C used to transmute thorium into fissile Uranium-233.[10] The next layer consists of heavy water at 20°C.[10] Farther inward is the pumped fuel salt layer (about 200 liters of LiF and UF4/3), which enters the reactor at 600°C and exits the reactor at 700°C, and serves as both fuel and core coolant.[10] The innermost layer is the moderator, more heavy water at 20°C, with the total amount of heavy water being ~1,200 liters.[10] The layers are separated by carbon composites.'
Initial cores, including the first commercial ones, will be stainless steel. Composite construction, very highly enriched lithium7, transfer of bred uranium from the blanket to the core, and removal of non-volatile fission products, are later goals to achieve breeding. Insulation between hot salt and D2O is 2-3 cm of yttrium-stabilised zirconia - most heating of the moderator is from neutrons, not conduction. From what I understand, Candu reactors are quite good for burning higher actinides - heavy water takes a lot more collisions to slow a neutron, so there's a fairly wide spectrum, including U238 absorption resonances at 10eV to 1 keV. CopAtomx propose moving to spent fuel actinides as a starter, after using low-enriched 235U to begin with.
A rival Danish molten salt startup (!), Seaborg Technologies, had wanted to use molten sodium hydroxide as a moderator, with High Assay LEU, but defaulted to graphite and LEU in 2023. Don't know their salt composition. No dates for fission trials.
John O'Neill
NNadir
(35,057 posts)...reactors and dismantle the many thousands of wind turbines in that offshore oil and gas drilling hellhole, and restore all the ecosystems littered with wind based industrial parks.
That, however, would make sense.
I have given a lot of thought over the years to layered reactor designs, albeit fast spectrum cases involving liquid metal fuels. I hope I can get my son to listen to them, as they involve some very interesting ceramic composites to perhaps inspire the material scientist in him. His graduate work is in metal alloys for structural purposes however, not fuels.