Environment & Energy
Related: About this forumContinuous Hydrolysis of CuCl2 in the Copper Based Thermochemical Hydrogen Cycle
The paper to which I will very briefly refer is this one: Continuous CuCl2 Hydrolysis in the Six-Step Cu–Cl Thermochemical Cycle for Green Hydrogen Production Ramdas S. Kadam, Ashwini B. Nirukhe, and Ganapati D. Yadav Industrial & Engineering Chemistry Research 2024 63 (48), 20787-20799.
I have low tolerance for all the bullshit handed out about "green" hydrogen, because usually "green" - a much abused word these days - is connected with so called "renewable energy," a mass and land intensive scheme popularly described as "green," although it is no such thing. The vast sums of money squandered on so called "renewable energy" has been useless, since so called "renewable energy" depends on access to fossil fuels, and thus has had no effect on arresting or slowing the extreme global heating we now observe. In fact things are getting worse faster.
I especially hold in contempt the tiresome, unworkable and frankly dangerous old idea of treating hydrogen as a consumer fuel; this said it is a valuable captive chemical intermediate when handled by highly trained chemical engineers in industrial plants.
There are thousands upon thousands of papers on thermochemical hydrogen production in the literature, across a vast array of hydrogen cycles. The most popular of these is the sulfur iodine cycle, and modifications thereof, which I personally prefer given it uses only liquid and gas phase molecules, and thus, at least in theory is capable of continuous operation in a closed system. The paper here refers another well known cycle the CuCl2 cycle, which also features many permutations (including the change of the halides from chlorine to bromine or iodine). The particular cycle discussed here uses electrolysis to generate chlorine, something I generally do not applaud, although with appropriate heat networks to employ process intensification, it may be possible to continuously produce excess electricity for which no immediate use is available other than electrolysis.
In modern times, perhaps to get grants, thermochemical cycles are often proposed using solar thermal garbage to provide the heat for reactions, since obeisance to the worship of so called "renewable energy" has become an element of quasi-religious faith that is regrettably widely spread throughout the world, despite the fact that it does nothing useful generating far more complacency than energy, complacency that has left the world in flames.
What is notable about this paper is how it defines "green," which is largely consistent with how I define it.
To wit, from the introduction of the paper:
Hydrogen as a clean energy source has the potential to be a viable replacement for traditional fossil fuels. Hydrogen usage on a large scale will require sustainable, low-cost, and environmentally friendly methods of production. (1,2) If hydrogen is produced using renewable energy sources, it can reduce greenhouse gas emissions (GHG) that contribute to climate change. Cu–Cl cycles represent promising water-splitting techniques that can be integrated, for instance, with nuclear reactors among other green energy sources. These cycles employ thermal processes to decompose water into oxygen and hydrogen, utilizing a series of intermediate reactions. Importantly, these cycles ensure the recycling of all other intermediate chemicals and result in zero emissions being released into the environment. The Cu–Cl cycle is an attractive alternative for hydrogen production by thermochemical water breakdown because it requires lower temperatures and has greater overall efficiency than other thermochemical cycles. (3,4)...
There are several dubious locutions in the introduction, and one should note the genuflection in the direction of "other green energy sources" of which there are effectively none.
Table 1 gives the reaction series for the CuCl2 cycle and the temperatures at which the reactions take place:
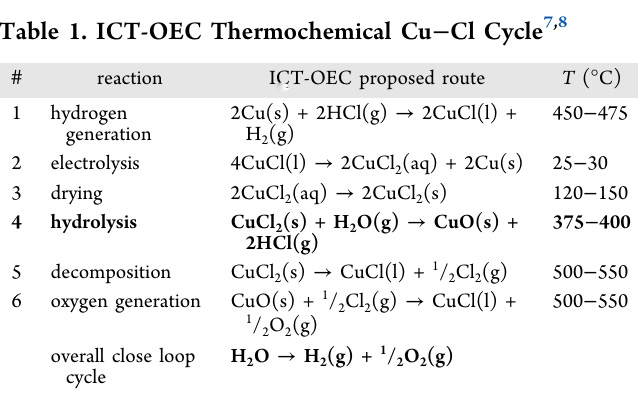
A few graphics from the text beginning with a flow diagram:
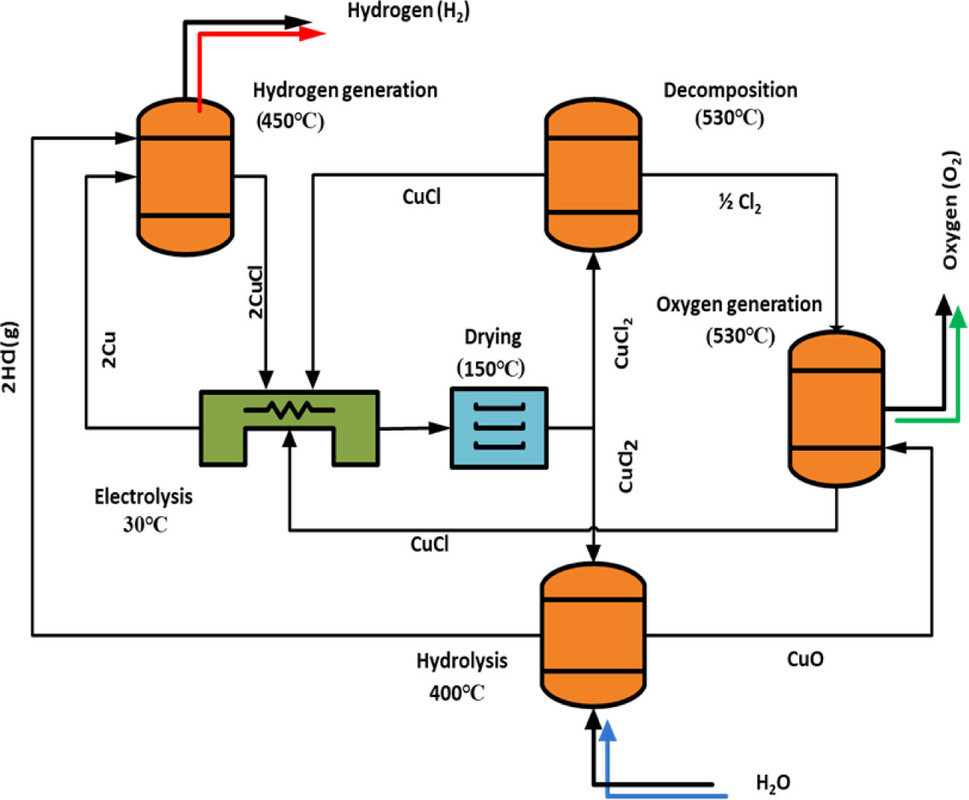
The caption:
A point relevant to the control of particle size:
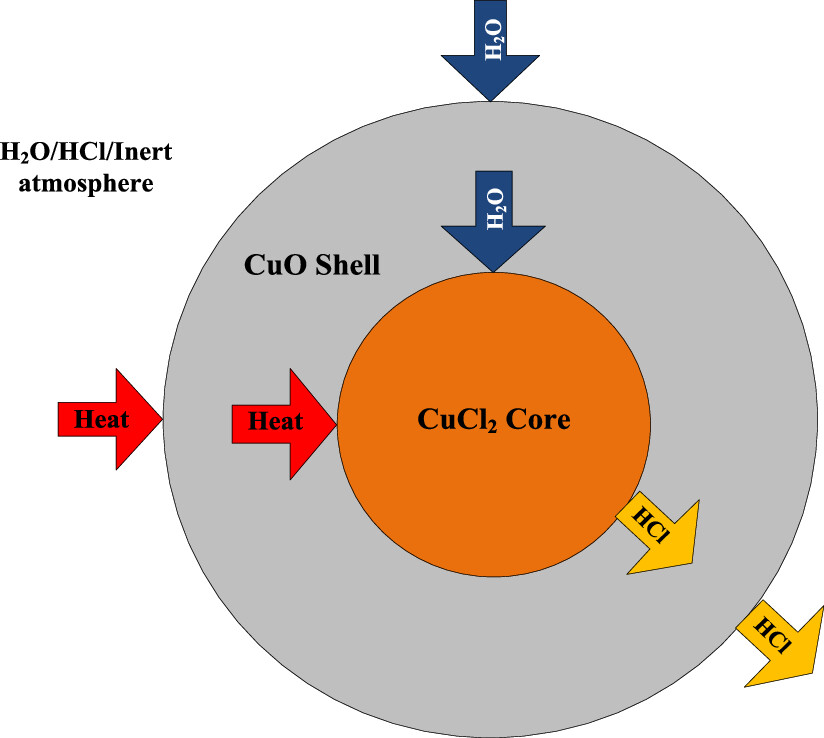
The caption:
A schematic for a reactor for continuous flow of the CuCl2 hydrolysis reaction.
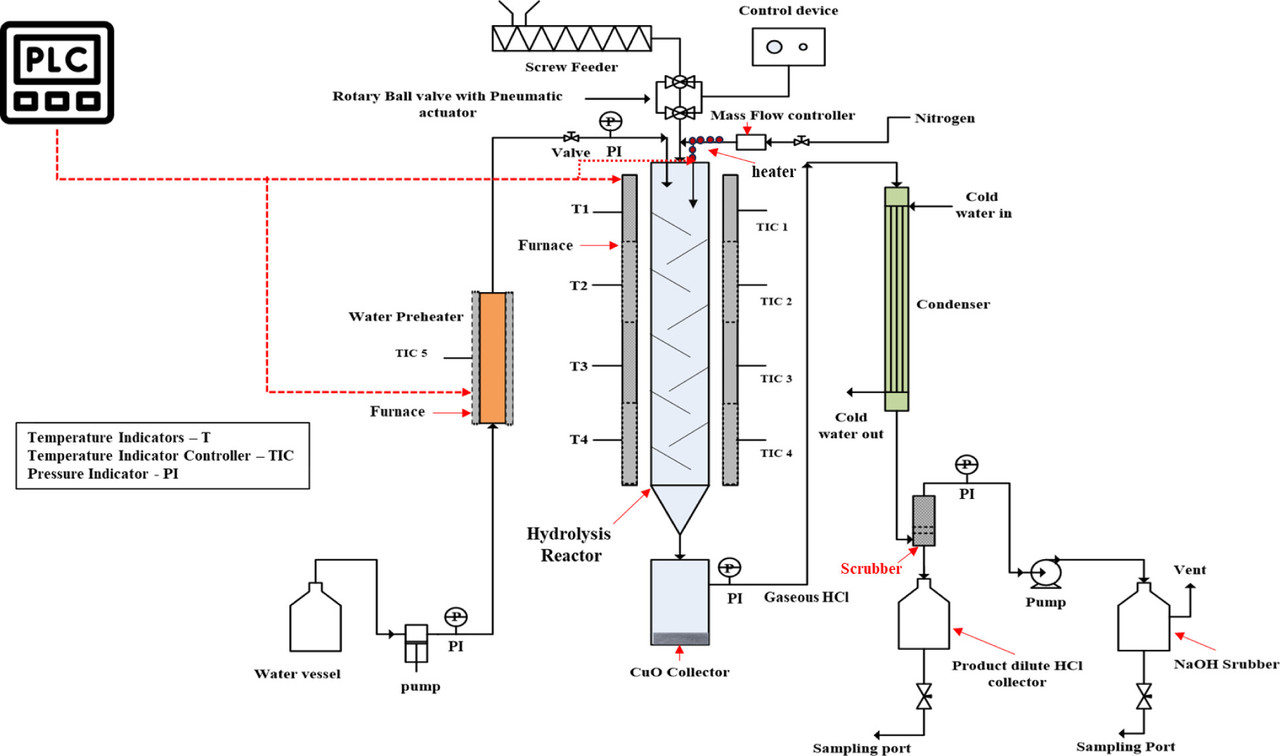
The caption:
Again, I'm not really a CuCl2 thermochemical cycle kind of guy, but what I appreciate is that the article delineates, in the way I do, what "green" might really be.
Have a nice weekend.
Bernardo de La Paz
(60,320 posts)NNadir
(37,349 posts)...correction to your post is that if you precipitated "copper chloride," it was not CuCl2, but CuCl, the monovalent chloride, which is analogous in its chemistry to silver (a congener of copper) which also forms insoluble monovalent halides, AgCl, AgBr, and AgI, salts involved in the discovery of photography. CuCl2 is soluble. It is possible the experiment you performed was a reduction of CuCl2 to CuCl, in which case the experiment you described was in fact what you did.
Interestingly, AgF is soluble, something I didn't know until I almost advised a junior scientist to use explain an analytical chemistry result and looked into it, happily, before hand.
Silver exhibits a +2 oxidation state only under extreme conditions, often involving fluorine as the oxidizing agent, although other examples are known.
(cf. Wojciech Grochala, Beyond fluorides: Extension of chemistry of divalent silver to oxo ligands, Inorganic Chemistry Communications, Volume 11, Issue 2, 2008.)
By contrast with its congener, the +2 oxidation state for copper is its most stable oxidation state.
Even more interesting is that Au, the other congener, prefers the +3 oxidation state, although it exhibits a monovalent state and although it is famously resistant to oxidation from its most stable oxidation state, zero, the metallic state, which is why alchemists in primitive times were so obsessed with it, because it was believed to contain the secret of eternal life.
It is a shame that kids no longer have interesting chemistry sets, if they have chemistry sets at all. I had one when I was a kid, and spent all of my time that I remember with it trying to make gun powder in a (thankfully) stupid way. My parents couldn't advise me because neither of them finished high school. If they had been exposed to chemistry they almost certainly would have declined to help me in the task.
A bit of irony:
As an old man, my father, became an amateur magician to put on shows for kids in hospitals, shelters and the like. Somehow got his hands of white phosphorous, and asked me about it, whereupon I freaked out that he was playing with it.
Thanks for your comment.
Bernardo de La Paz
(60,320 posts)By "precipitated" I should have written "precipitated from".
I'm pretty sure now it was copper sulphate and it was most likely a sodium carbonate solution that I added.
NNadir
(37,349 posts)adventures is my failed method to make gun powder, which I attempted to do in aqueous solution.
Of course, my chemistry set adventures took place probably over six decades ago, and I really had no idea of what chemistry was all about. My chemistry career began when I decided to become a pharmacist, after working as a pharmacy tech in a hospital as a kid (where I filled prescriptions with some supervision), and after taking my first perquisite chemistry class I realized that I could already count to 100, type labels off Latin abbreviations, and thus decided to do something more interesting.
That too, was a very long time ago.
Think. Again.
(22,456 posts)...as noted in the snippet you quoted, and so your insistence that...
"The vast sums of money squandered on so called "renewable energy" has been useless, since so called "renewable energy" depends on access to fossil fuels, and thus has had no effect on arresting or slowing the extreme global heating we now observe. "
...is, as always, wrong.